Medtronic
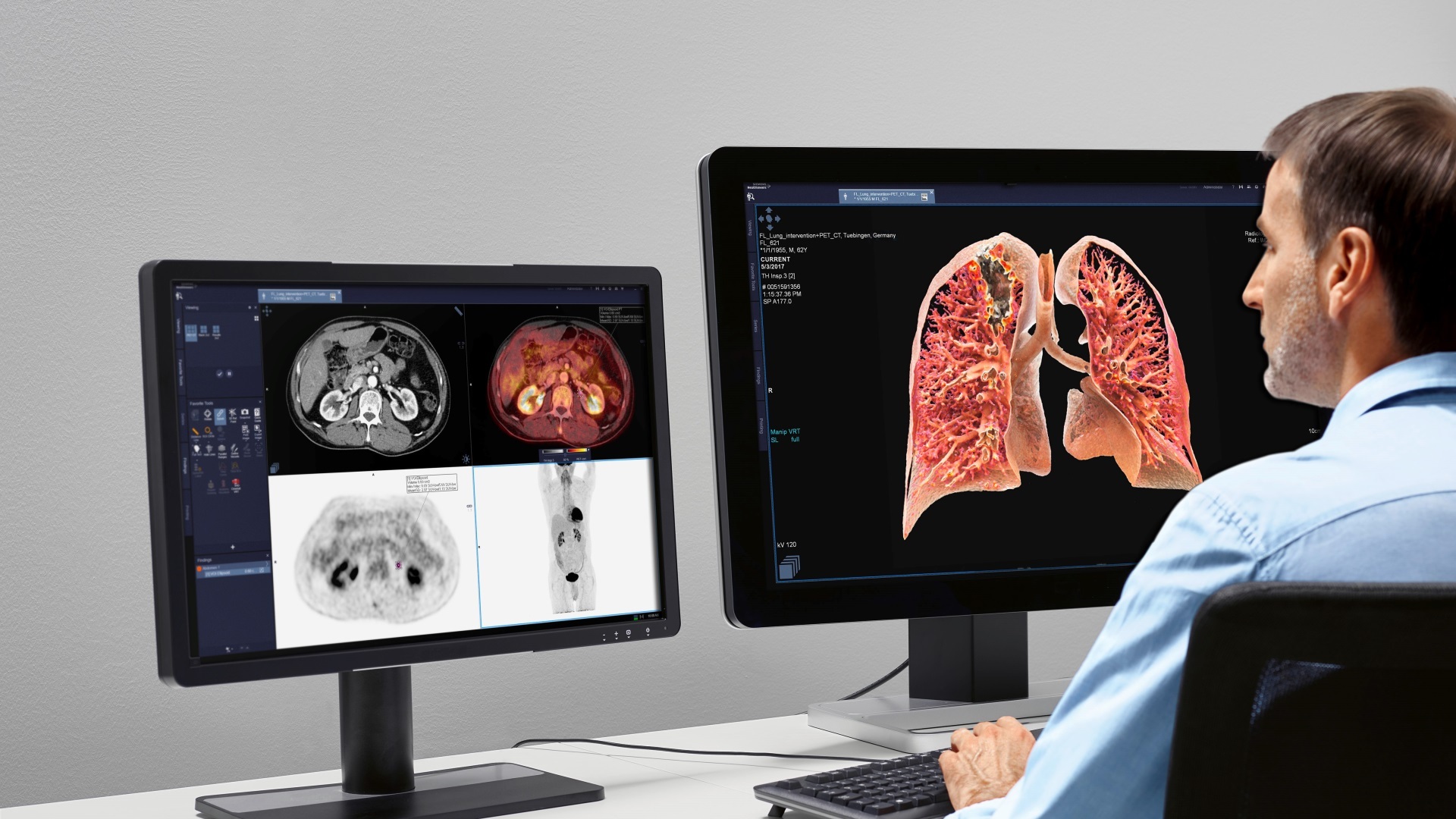
Medtronic, a leader among medical technology providers, has announced the United States Food and Drug Administration’s approval of the Medtronic Concerto/Virtuoso line of implantable cardiac devices. Market availability of these devices will follow in June 2006. The Concerto cardiac resynchronisation therapy-defibrillator (CRT-D) and Virtuoso implantable cardioverter defibrillator (ICD) are the first implantable cardiac devices available with Medtronic’s proprietary Conexus Wireless Telemetry, developed using the Medical Implant Communications Service (MICS, 402-405 MHz). Conexus Telemetry will enable automatic, wireless data transmission from the patient’s device to a home monitor. Medtronic Conexus Telemetry enables reliable communication between the patient’s implanted device and home monitor or clinician programmer at a range of two to five meters (approximately six to 16 feet).

post_id:
uld_count:
Cookie not set
Value 1: 0
Value 2: 10